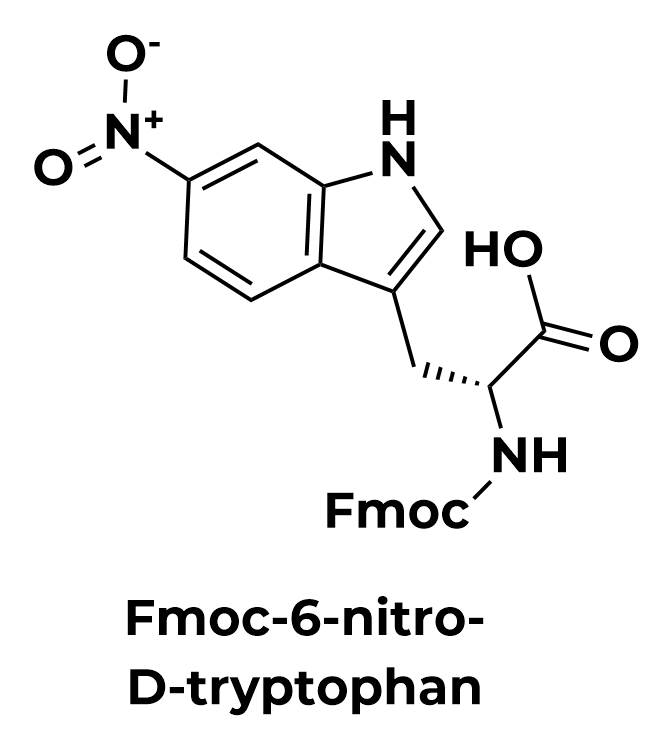
Enantiopure D-amino Acid Analogs Made Using Enzymes
As peptide therapeutics experience explosive growth, the need to address the pharmacokinetic liabilities of canonical L-amino acids grows. D-amino acids are less susceptible to enzymatic degradation and, when placed appropriately in a peptide or protein sequence, can confer additional selectivity and potency.
Combining the D-enantiomeric space with the versatile Aralez Bio functional group and positional space leads to an expansive chemical diversity with endless possibilities.
Incorporate D-amino acids into your workflow to:
- screen expanded chemical space
- improve protease stability
- improve binding affinity & specificity
- design molecules from mirror image display
- optimize peptide-target interactions
- perform racemic crystallography
- identify key interactions with D-amino acid scanning
- alter secondary structure
- drive self-assembly